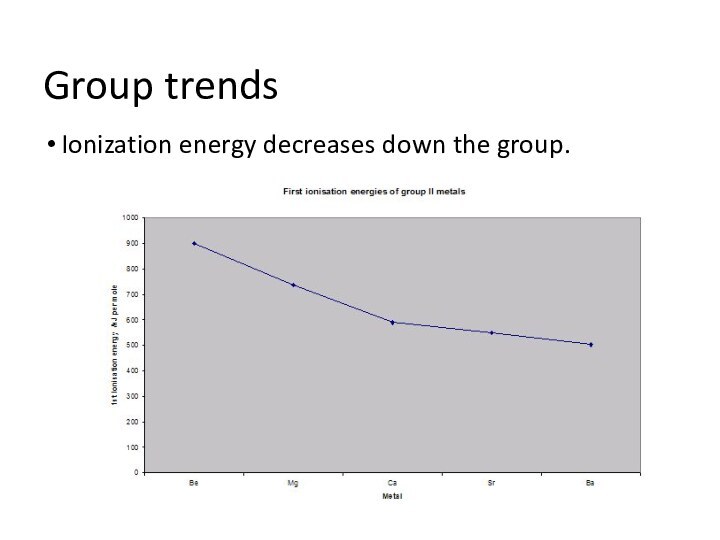
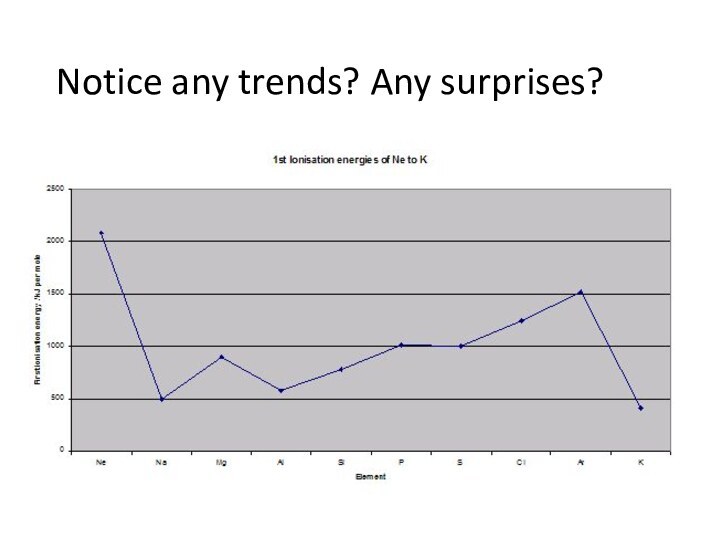
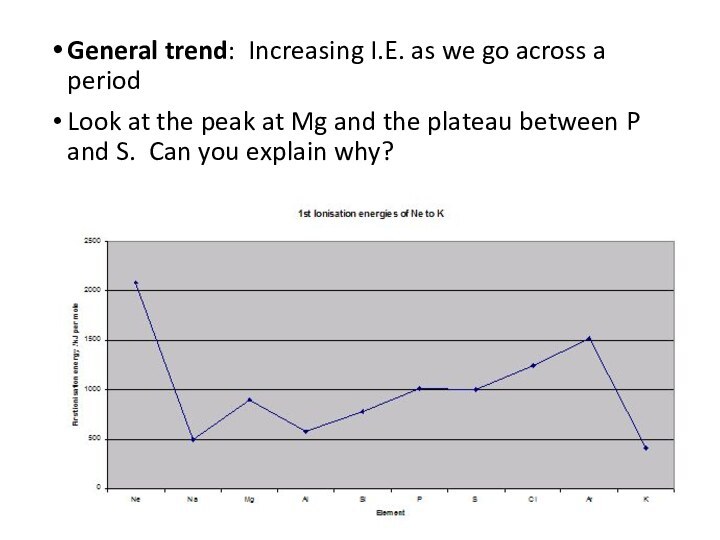
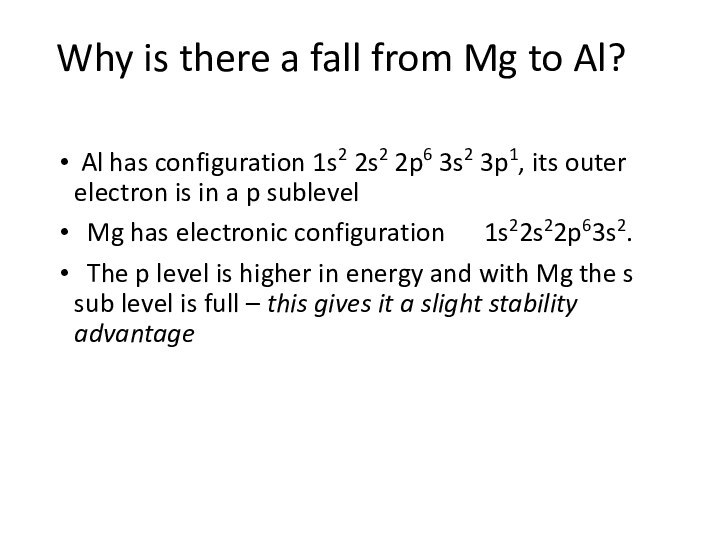
remove an electron from a gaseous atom.
An atom's 'desire'
to grab another atom's electrons. Removing one electron makes a +1 ion.
The energy required is called the first ionization energy.
X(g) + energy →X+ + e-