- Главная
- Разное
- Бизнес и предпринимательство
- Образование
- Развлечения
- Государство
- Спорт
- Графика
- Культурология
- Еда и кулинария
- Лингвистика
- Религиоведение
- Черчение
- Физкультура
- ИЗО
- Психология
- Социология
- Английский язык
- Астрономия
- Алгебра
- Биология
- География
- Геометрия
- Детские презентации
- Информатика
- История
- Литература
- Маркетинг
- Математика
- Медицина
- Менеджмент
- Музыка
- МХК
- Немецкий язык
- ОБЖ
- Обществознание
- Окружающий мир
- Педагогика
- Русский язык
- Технология
- Физика
- Философия
- Химия
- Шаблоны, картинки для презентаций
- Экология
- Экономика
- Юриспруденция
Что такое findslide.org?
FindSlide.org - это сайт презентаций, докладов, шаблонов в формате PowerPoint.
Обратная связь
Email: Нажмите что бы посмотреть
Презентация на тему Electron Structure
Содержание
- 2. Atomic StructureProtons, neutrons, electronsHow to make ionsRelative atomic mass
- 4. 0-1+111200019.109 x 10-311.602 x 10-191.672 x 10-271.602
- 5. Ionisation EnergyWhat is ionisation energy?DefinitionsFirst ionisation energySuccessive ionisation energiesWhat affects ionisation energy?
- 6. WHAT IS IONISATION ENERGY?Ionisation Energy is a
- 7. WHAT AFFECTS IONISATION ENERGY?The value of the
- 8. Ionisation Energy is affected by 3 things:Atomic
- 9. Successive Ionisation EnergiesA measure of the energy
- 10. Which electron is removed first?(First Ionisation Energy)
- 11. Which electron is removed first?(First Ionisation Energy)
- 12. Successive Ionisation Energies of CalciumDraw a graph
- 15. 1s2 2s2 2p6 3s2 3p6 4s22,8,8,2
- 16. Скачать презентацию
- 17. Похожие презентации
Atomic StructureProtons, neutrons, electronsHow to make ionsRelative atomic mass












Слайд 4
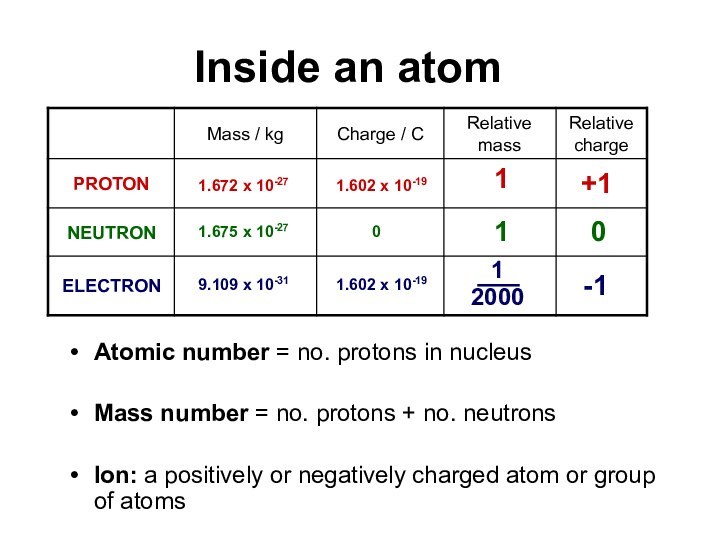
0
-1
+1
1
1
2000
1
9.109 x 10-31
1.602 x 10-19
1.672 x 10-27
1.602 x
10-19
1.675 x 10-27
0
Inside an atom
Atomic number = no. protons
in nucleusMass number = no. protons + no. neutrons
Ion: a positively or negatively charged atom or group of atoms
Слайд 5
Ionisation Energy
What is ionisation energy?
Definitions
First ionisation energy
Successive ionisation
energies
What affects ionisation energy?
Слайд 6
WHAT IS IONISATION ENERGY?
Ionisation Energy is a measure
of the amount of energy needed to remove electrons
from atoms.As electrons are negatively charged and protons in the nucleus are positively charged, there will be an attraction between them. The greater the pull of the nucleus, the harder it will be to pull an electron away from an atom.
FIRST IONISATION ENERGY - Definition
The energy required to remove ONE MOLE of electrons from each atom in ONE MOLE of gaseous atoms to form ONE MOLE of gaseous positive ions.
e.g. Na(g) Na+(g) + e-
Al(g) Al+(g) + e-
Make sure you write in the (g)
Слайд 7
WHAT AFFECTS IONISATION ENERGY?
The value of the 1st
Ionisation Energy depends on the electronic structure
Hydrogen
Helium
Lithium
The value for
helium is higher than that for hydrogen because there are now two protons in the nucleus. The nuclear charge is greater so the pull on the outer electrons is larger. More energy will be needed to pull an electron out of the atom.519 kJ mol-1
1310 kJ mol-1
2370 kJ mol-1
Слайд 8
Ionisation Energy is affected by 3 things:
Atomic Radius
Nuclear
Attraction
Electron Shielding
I. E. Decreases
I. E. Decreases
I. E.
Increases
Слайд 9
Successive Ionisation Energies
A measure of the energy required
to remove each electron in turn.
Mg(g) ? Mg+(g) +
e- 1st I.E. =+738 kJ.mol-1Mg+(g) ? Mg2+(g) + e- 2nd I.E.= + 1451kJ.mol-1
Mg2+(g) ? Mg3+(g) + e- 3rd I.E.= + 7733kJ.mol-1
Mg3+(g) ? Mg4+(g) + e- 4th I.E.= + 10541kJ.mol-1
Слайд 12
Successive Ionisation Energies of Calcium
Draw a graph to
show the successive ionisation energies of calcium, using the
log10 values(press log, then the number, then = )
Explain all the main points about the graph that you can see (use Pg 41 of OCR AS Chemistry to help you)