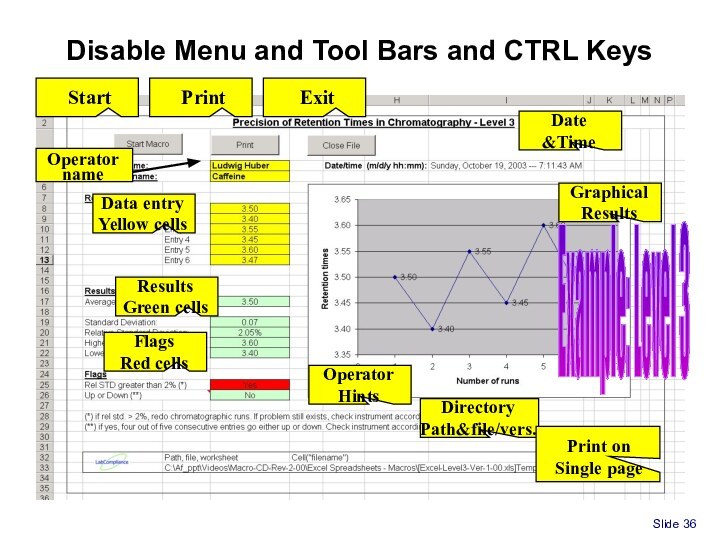
Spreadsheet for Part 11/GxP compliance
Validation during development and installation
How
to ensure integrity&security of spreadsheetsHow the FDA is using spreadsheets
Documentation requirements
Validation example from beginning to the end
Case studies for Part 11 compliance
Q&A's
Q&A's
Part 1
Part 2